Forschen an der THD
Innovativ & Lebendig
IMSE is an inclusive community developing new research paradigms and curricula that bring together engineering, science and medicine to advance personalised precision medicine and train people to seamlessly connect these historically separate fields.
Research focus
Precise surgical planning through personalised simulation
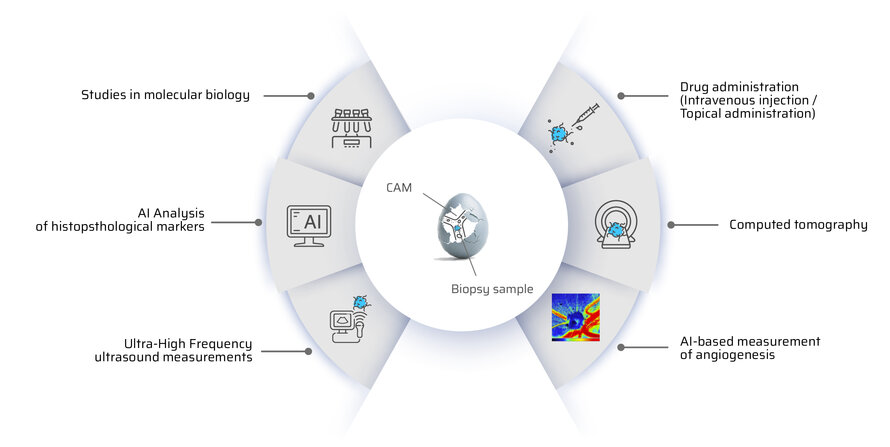
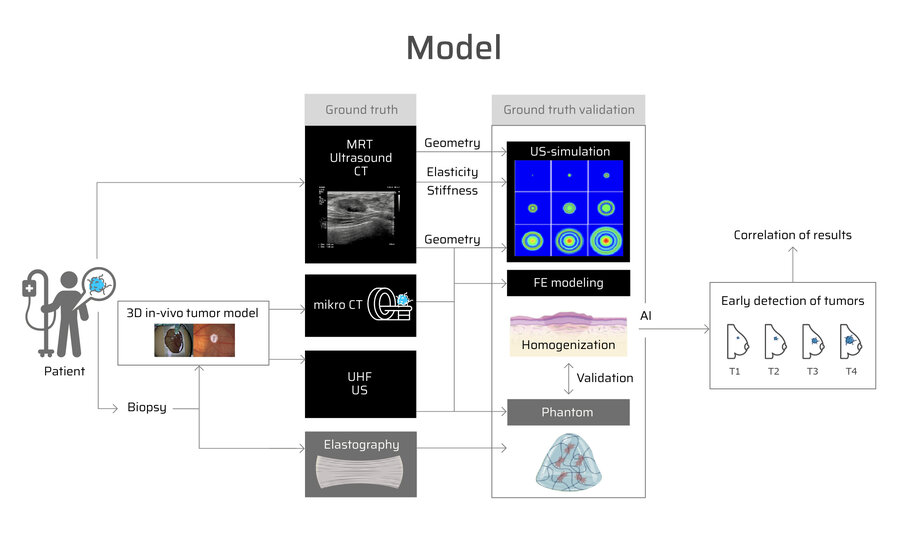
How can tumour surgery be optimally prepared? Our approach combines state-of-the-art imaging, tissue cultivation and simulation to support individualised treatment decisions.
A patient's tumour is diagnosed using computed tomography (CT), ultrasound (US) and magnetic resonance imaging (MRI). A biopsy provides tumour tissue, which is cultivated on the chorioallantoic membrane (CAM). During growth, regular CT, US and elastography examinations are performed to analyse tissue stiffness, volume changes and the response to therapy.
At the same time, we simulate the behaviour of the tumour during radiotherapy and chemotherapy. Our models show how well the treatment responds, how quickly the tumour grows and whether vascular or nerve-sparing surgical procedures are possible. These precise predictions help to plan surgical interventions in a more targeted manner and ensure the best possible treatment for the patient.
projects
We bring applied research to companies. From the idea to industrial application.
Would you like to work with us on a publicly funded project to put your ideas into practice? Then we look forward to receiving your enquiry.
Your benefits:
- Secure funding for research projects (SMEs up to 80 %)
- Low administrative requirements from your side. We take over the process of coordinating application activities and organising ongoing projects
- Maximising economic efficiency - optimised manufacturing processes, improved material selection
- Carry out joint research work in a practical and scientifically based manner
- Open-topic research in the field of materials science and process analysis
- Prototypes are developed to market maturity in joint projects and the potential for business start-ups is explored.
We offer a wide range of services for solving material science problems.
We take individual requirements into account and rely on customised approaches that are supported by state-of-the-art testing technologies, simulations and AI.
- Smart3D - Smart 3D printing of high-performance polymer systems
Partners: TC Grafenau, Ingenieurbüro Muhr GmbH, Kumovis GmbH, nebumind GmbH - SensoTwin - Sensor-integrated digital twin for high-performance fibre composite applications
Partners: TC Freyung, TUM, TÜV Süd Industries Service GmbH, Enercon, Fos4x, UL International GmbH - AutoClean - Development of an automated post-processing line with integrated AI-based quality control and sorting station
and sorting station for components from powder bed-based 3D printing
Partners: TC Cham, SHL Automatisierungstechnik AG, thinkTEC3D GmbH - Powercube - Deployables Cubed GmbH - This project is about the development of a mini-satellite, which should deliver a power of 100W until the end of the mission. Furthermore, the structure is to be designed in such a way that it can be stowed in the volume of a cube with an edge length of 10 cm, can be transported safely, unfolds easily at the mission target and does not weigh more than two kilograms. In order to comply with the specified boundary conditions, the implementation of an integral fibre-reinforced carrier structure with innovative joints is being investigated. Deployment simulations and tests are being carried out to validate the concepts.
- HiPower - Industrial research and construction of a prototype for a novel 100W high power solar panel subsystem for New Space NanoSats and SmallSats Partner: Deployables Cubed GmbH
- EXRE - Applied exoskeleton research for use in rehabilitation. The project aims to improve synergies between the regions in applied research. An international, interdisciplinary research team is driving the development of an exoskeleton prototype for rehabilitation and prosthetic practice.
Joint innovations in the fields of design, construction technologies, 3D printing, mechatronics, applied cybernetics (brain-computer interface) and rehabilitation will be utilised. The participation of 5 associated partners from the field and the testing of the exoskeleton enable a transfer of expertise to SMEs in the fields of mechatronics, rehabilitation and prosthetics.
Partner: University of West Bohemia: ZČU in Pilsen
- SurfMod3Dton - Functionalisation of 3D (FDM) components for detonation coating
Partner: Reimann Industrietechnik GmbH - Sumida Components & Modules GmbH - Effective chain analysis for injection moulded parts in high-performance electronic components In cooperation with Sumida, the Technology Campus Hutthurm is developing a simulation strategy to transfer the results from the injection moulding simulation to a structural simulation. The subsequent FE calculation is intended to optimise the load-bearing behaviour while simultaneously improving the component design.
- ForCEs - Exoskeleton research cluster - cyber-physical systems for the working world of the future
Partners: TC Freyung, TC Cham
Current Student projects
Abstract:
Hepato-pancreato-biliary (HPB) tumors exhibit high aggressiveness, invasiveness, and therapy resistance. Despite advancements like mFOLFIRINOX, survival rates remain low, and optimal treatment strategies are still undefined. This study integrates AI-based analysis and a 3D in-vivo tumor model to assess the effect of potential therapeutics on histopathological markers. The patient-specific chorioallantoic membrane (CAM) model offers an alternative to animal testing, enabling the cultivation of human tissues and drug testing. For the first time, a combination of Sunitinib and Gemcitabine will be tested in the CAM model, building on prior mouse studies demonstrating superior antitumor efficacy. Histological and immunohistochemical analyses will be performed using markers such as Ki-67, p53, CD3, and CD20 to investigate the proliferation rate, mutation rate, immune response and tumor stroma ratio. AI-assisted tools like QuPath will be employed to enhance digital pathology and automate biomarker quantification. This approach aims to optimize specificity, reduce manual correction efforts, and improve
prognostic assessments. The findings will contribute to a better understanding of therapeutic efficacy, resistance mechanisms, and the potential for personalized treatments.
Abstract:
Die autosomal dominante polyzystische Nierenerkrankung (ADPKD) ist die häufigste Form einer monogenetischen Nierenerkrankung. Die Krankheit ist durch eine fortschreitende, bilaterale Entwicklung und Vergrößerung von Zysten gekennzeichnet, wodurch umliegendes Gewebe verdrängt wird. Infolgedessen benötigen etwa 50 % der ADPKD-Patienten bis zum Alter von 55 Jahren eine Nierenersatztherapie. Da es sich bei ADPKD um eine eher langsam fortschreitende Krankheit handelt, die sich oft über Jahrzehnte entwickelt, bleibt ein großer Zeitrahmen, um das Zystenwachstum zu hemmen und die Nierenfunktion zu erhalten. ADPKD wird hauptsächlich durch heterozygote Mutationen des PKD1- oder
PKD2-Gens verursacht. Die genauen molekularen Mechanismen sind noch nicht vollständig geklärt. Ein besseres Verständnis der Bildung und des Wachstums von Nierenzysten bei ADPKD wäre für die Umsetzung neuer Behandlungsziele und Strategien zur Erhaltung der Nierenfunktion bei diesen Patienten von großem Wert. Derzeit gibt es keine ausreichenden Therapieansätze, um das Zystenwachstum zu inhibieren oder zu verlangsamen und so die Nierenfunktion bei der ADPKD zu erhalten. Der Bedarf solcher Therapieansätze ist aber sehr hoch. Der für die Therapie von ADPKD verwendete Vasopressin 2 Rezeptor Antagonist Tolvaptan (Jinarc®), ist bislang die einzige und auch nur in einigen Ländern
zugelassene Substanz zur Hemmung des Zystenwachstums. Da der Effekt auf das Zystenwachstum nur schwach ausgeprägt ist und von relevanten Nebenwirkungen begleitet wird, ist die Suche nach alternativen therapeutischen Ansätzen unumgänglich. Ein bekanntes Problem bei ADPKD ist die Übertragung von Ergebnissen aus nicht-humanen Modellen auf den Menschen. Das 3D-in-vivo-Modell (Chorioallantoismembran-Modell) kann als Zwischenschritt zwischen Tierversuchen und Studien am Menschen dienen. Als wesentlicher Zwischenschritt zu einer
potentiellen klinischen Anwendung sollen daher vielversprechende Inhibitoren an humanem Zystengewebe in einem 3D-Modell getestet werden. Die Verwendung von menschlichem Zystengewebe stellt nicht nur einen weiteren Schritt in Richtung Translation dar, sondern trägt auch zur Implementierung der 3R-Prinzipien („reduce, refine, replace“) durch Einsatz von Alternativmethoden zum Tierversuch bei.
Abstract:
Pancreatic ductal adenocarcinoma (PDAC) and cholangiocellular carcinoma (CCA) share aggressiveness, short survival time and resistance to chemotherapy. FOXO1 and microRNA-21 function respectively as tumor suppressor and oncogene in PDAC. Moreover, FOXO1 has an important role in vascular homeostasis because it is a fundamental modulator of the formation and maturation of blood vessels.
The cell line with the lowest FOXO1 expression will be selected for transfection. A first transfection experiment will involve the overexpression of FOXO1. Once the transfection is confirmed through western blot and genomic DNA genotyping, the clone will be inoculated onto the CAM. IKOSA will be used to monitor the vessels in the CAM. Part of the explanted cell pellets will be used to analyse the expression angiogenic factors and for immunohistochemistry for the staining of FOXO1 and phospho FOXO1Ser256 (phosphorylated FOXO1). As part of preliminary work, the protein expression of FOXO1 was detected using Western blotting in different PDAC and CCA cell lines. The expression of FOXO1 was significantly higher in BxPC-3 than in MiaPaCa2. Therefore, MiaPaCa2 is a good candidate for overexpression of the gene, while BxPC-3 is a good candidate for knockdown experiments. In addition, cholangiocellular carcinoma tissue was cultured on the CAM model for the first time and the macroscopic course was closely analyzed over a period of one week. The vascularization of CCA onto the CAM was confirmed through ultra-high-frequency ultrasound measurements and we observed the proliferation of the tissue after growth on the CAM using Ki67 staining. The aims are investigating the role of FOXO1 and microRNA-21 in angiogenesis of PDAC and CCA; and cultivating dCCA tumor tissue on the CAM for testing chemotherapeutic drugs.
Team
- Prof. Dr. med. habil. Thiha Aung (Director)
- Prof. Dr. med. Katharina Schilbach
- Prof. Dr. med. Michael Frey
- Prof. Dr. Silke Härteis
- PD Dr. med. Dr. med. dent. Tolga Taha Sönmez
- PD Dr. med. Martin Kammerl
- PD Dr. Katharina Schmidt
- Dr. med. univ. Bettina Huber
- Prof. Dr. med. Christina Hackl
- TA: Eva Wirkert
- Agata Montagner
- Laura Lemberger
- Jan Schüler
- Lea Kiefer
- Nandar Lamin Aye
- Christiane Loibl
- Bengisu Alay
- Prof. Sebastian Kölbl (Director)
- Prof. Dr. Florian Wahl
- Prof. Dr. Simon Zabler
- Prof. Dr.-Ing. Thomas Spittler
- Prof. Dr. Matthias Hien
- Prof. Dr. Christoph Schober
- Prof. Dr. Matthias Hien TC Cham
- Prof. Dr. med. habil. Thiha Aung: PhA, MBA, Kosmetkwissenschaften
News


We were delighted to welcome Prof. Dr. Chris Lim from Massachusetts General Hospital and Harvard University to the Hutthurm campus.
The focus was on joint research projects at the interface between medicine and engineering, as well as an exchange on teaching in the Physician Assistant programme. Prof. Lim learned about current activities on campus, including innovative approaches to improving care for diabetes patients and chronic wounds.
The discussions on practical approaches, international expertise and the transfer of modern research directly to the region were particularly exciting. It became clear how important the connection between engineering and medicine is – for example, in the development of personalised solutions using 3D printing that can improve patient treatment.
The visit once again highlighted the potential of applied research, the transfer of knowledge into practice and the opportunities for regional healthcare.
Directions
THD - Technische Hochschule Deggendorf
Dieter-Görlitz-Platz 1
94469 Deggendorf
E-Mail: imse@th-deg.de








